Molar Mass Of Ammonium Sulfide
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Ammonium ion | |||
| Systematic IUPAC name Azanium[1] | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| ChEBI |
| ||
| ChemSpider |
| ||
| MeSH | D000644 | ||
| PubChem CID |
| ||
| UNII |
| ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemical formula | [NHfour]+ | ||
| Tooth mass | 18.039 g·mol−one | ||
| Acerbity (pGrand a) | 9.25 | ||
| Conjugate base | Ammonia | ||
| Construction | |||
| Molecular shape | Tetrahedral | ||
| Except where otherwise noted, data are given for materials in their standard land (at 25 °C [77 °F], 100 kPa). Infobox references | |||
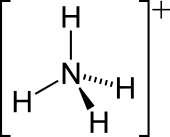
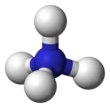
The ammonium cation is a positively-charged polyatomic ion with the chemical formula NH + 4 or [NHfour]+ . It is formed past the protonation of ammonia (NH3 ). Ammonium is also a full general proper noun for positively charged or protonated substituted amines and quaternary ammonium cations ([NRfour]+ ), where one or more hydrogen atoms are replaced by organic groups (indicated past R).
Acid–base properties [edit]

The ammonium ion is generated when ammonia, a weak base of operations, reacts with Brønsted acids (proton donors):
The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule:
Thus, treatment of concentrated solutions of ammonium salts with stiff base gives ammonia. When ammonia is dissolved in water, a tiny corporeality of it converts to ammonium ions:
The caste to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions. If the pH is high (the concentration of hydrogen ions is low and hydroxide ions is high), the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia.
Formation of ammonium compounds can too occur in the vapor stage; for example, when ammonia vapor comes in contact with hydrogen chloride vapor, a white cloud of ammonium chloride forms, which eventually settles out as a solid in a sparse white layer on surfaces.
Salts [edit]

Ammonium cation is found in a variety of salts such as ammonium carbonate, ammonium chloride and ammonium nitrate. Well-nigh simple ammonium salts are very soluble in water. An exception is ammonium hexachloroplatinate, the formation of which was once used as a examination for ammonium. The ammonium salts of nitrate and peculiarly perchlorate are highly explosive, in these cases ammonium is the reducing agent.
In an unusual process, ammonium ions grade an amalgam. Such species are prepared by the addition of sodium amalgam to a solution of ammonium chloride.[2] This constructing eventually decomposes to release ammonia and hydrogen.[3]
To detect whether the ammonium ion is nowadays in the salt, kickoff the salt is heated in presence of alkali hydroxide releasing a gas with feature olfactory property which of class is ammonia.
To further confirm ammonia it passed through glass rod dipped in HCl solution (hydrochloric acid) creating white dense fumes of ammonium chloride.
Ammonia when passed through CuSO4 (copper(Two) sulphate) solution turns from blue to deep blue color forming Schweizer'south reagent.
Ammonia or ammonium ion when added to Nessler's reagent gives brown color precipitate known as iodide of Million'south base in bones medium.
Ammonium ion when added to chloroplatinic acid gives a yellow precipitate.
Ammonium ion when added to sodium cobaltinitrite gives a yellow precipitate.
Ammonium ion when added to potassium bitartrate gives a white precipitate.
Construction and bonding [edit]
The lone electron pair on the nitrogen atom (N) in ammonia, represented equally a line to a higher place the N, forms the bond with a proton (H+ ). Thereafter, all four N–H bonds are equivalent, being polar covalent bonds. The ion has a tetrahedral structure and is isoelectronic with methane and borohydride. In terms of size, the ammonium cation (r ionic = 175 pm)[ citation needed ] resembles the caesium cation (r ionic = 183 pm).[ commendation needed ]
Organic ions [edit]
The hydrogen atoms in the ammonium ion tin exist substituted with an alkyl group or some other organic group to form a substituted ammonium ion (IUPAC nomenclature: aminium ion). Depending on the number of organic groups, the ammonium cation is called a primary, secondary, tertiary, or quaternary. With the exception of the fourth ammonium cations, the organic ammonium cations are weak acids.
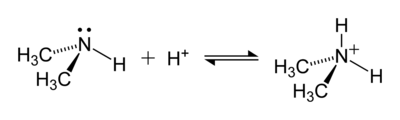
An instance of a reaction forming an ammonium ion is that between dimethylamine, (CH3)2NH, and an acrid to give the dimethylammonium cation, [(CH3)2NH2]+ :
Quaternary ammonium cations have 4 organic groups attached to the nitrogen atom, they lack a hydrogen atom bonded to the nitrogen atom. These cations, such every bit the tetra-north-butylammonium cation, are sometimes used to supercede sodium or potassium ions to increment the solubility of the associated anion in organic solvents. Primary, secondary, and 3rd ammonium salts serve the same office, but are less lipophilic. They are as well used as phase-transfer catalysts and surfactants.
An unusual grade of organic ammonium salts are derivatives of amine radical cations, [R3N•]+ such as tris(4-bromophenyl)ammoniumyl hexachloroantimonate.
Biological science [edit]
Ammonium ions are a waste matter of the metabolism of animals. In fish and aquatic invertebrates, it is excreted directly into the water. In mammals, sharks, and amphibians, it is converted in the urea bike to urea, considering urea is less toxic and tin can be stored more efficiently. In birds, reptiles, and terrestrial snails, metabolic ammonium is converted into uric acrid, which is solid and can therefore be excreted with minimal water loss.[4]
Ammonium is an important source of nitrogen for many institute species, particularly those growing on hypoxic soils. Yet, information technology is also toxic to about crop species and is rarely applied as a sole nitrogen source.[5]
Metallic [edit]
The ammonium ion has very like properties to the heavier brine metals cations and is often considered a shut equivalent.[6] [7] [8] Ammonium is expected to behave as a metal ([NHfour]+ ions in a sea of electrons) at very high pressures, such as within gas giant planets such equally Uranus and Neptune.[vii] [8]
Under normal conditions, ammonium does non exist as a pure metal, simply does as an amalgam (blend with mercury).[9]
See too [edit]
- Ammonium transporter
- f-ratio
- Hydronium (H3O+)
- Iminium
- Nitrification
- Onium compounds
- The Magnificent Possession (Isaac Asimov short story)
References [edit]
- ^ International Matrimony of Pure and Applied Chemistry (2005). Classification of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (United kingdom): RSC–IUPAC. ISBN 0-85404-438-8. pp. 71,105,314. Electronic version.
- ^ "Pseudo-binary compounds". Archived from the original on 2020-07-27. Retrieved 2007-10-12 .
- ^ "Ammonium Salts". VIAS Encyclopedia.
- ^ Campbell, Neil A.; Jane B. Reece (2002). "44". Biology (sixth ed.). San Francisco: Pearson Educational activity, Inc. pp. 937–938. ISBN978-0-8053-6624-two.
- ^ Britto, DT; Kronzucker, HJ (2002). "NHiv + toxicity in higher plants: a critical review" (PDF). Journal of Institute Physiology. 159 (6): 567–584. doi:10.1078/0176-1617-0774.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemical science, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN0-12-352651-5
- ^ a b Stevenson, D. J. (November 20, 1975). "Does metallic ammonium exist?". Nature. 258 (5532): 222–223. Bibcode:1975Natur.258..222S. doi:10.1038/258222a0. S2CID 4199721.
- ^ a b Bernal, M. J. Thou.; Massey, H. S. Westward. (February 3, 1954). "Metal Ammonium". Monthly Notices of the Regal Astronomical Club. 114 (2): 172–179. Bibcode:1954MNRAS.114..172B. doi:10.1093/mnras/114.ii.172.
- ^ Reedy, J.H. (Oct i, 1929). "Lecture demonstration of ammonium amalgam". Journal of Chemical Education. 6 (10): 1767. Bibcode:1929JChEd...6.1767R. doi:10.1021/ed006p1767.
Molar Mass Of Ammonium Sulfide,
Source: https://en.wikipedia.org/wiki/Ammonium
Posted by: lewispaince.blogspot.com


![{\displaystyle {\ce {H+ + NH3 -> [NH4]+}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b09135f318a8156b2e420e7e8649d42fefa2bc51)
![{\displaystyle {\ce {[NH4]+ + B- -> HB + NH3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/919cc6fb00019fee68ccc7bcfa8214910853628f)
![{\displaystyle {\ce {H2O + NH3 <=> OH- + [NH4]+}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/631270c1ff852ddf6fc0ca7335761ec5874731d7)
![{\displaystyle {\ce {[NH4]+ + OH- ->[heat] NH3 + H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ddfc9836963f68746656202104369566b8520cd6)
![{\displaystyle {\ce {NH3_{(g)}{}+ HCl_{(aq)}-> [NH4]Cl_{(s)}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7d3eca1f053c4fdde9d569ee4a93828f0bba05a2)
2_{(aq)}{}+ H2SO4_{(aq)}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2210b7ab5b263f73ff03a5cb3ed4355d86a043ca)
![{\displaystyle {\ce {H2[PtCl6]_{(aq)}{}+ [NH4]+_{(aq)}-> [NH4]2[PtCl6]_{(s)}{}+ 2 H+}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5a75267fc6072184833d909420ff49c63951deba)
![{\displaystyle {\ce {Na3[Co(NO2)6]_{(aq)}{}+ 3 [NH4]+_{(aq)}-> [NH4]3[Co(NO2)6]_{(s)}{}+ 3 Na+_{(aq)}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6ab602493ba082c23d3c48ab02f82485d204634b)
![{\displaystyle {\ce {KC4H5O6_{(aq)}{}+ [NH4]+_{(aq)}-> [NH4]C4H5O6_{(s)}{}+ K+_{(aq)}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/97696c043f49cf51916efa72d98bdb61caab155c)

0 Response to "Molar Mass Of Ammonium Sulfide"
Post a Comment